13. Enthalpy and entropy changes of a reversible reaction are 40.63 kJ mol–1 and 108.8 J K–1 mol–1 respectively. Predict the feasibility of the reaction at 27^° C.
For the reaction A B, Δ H = –90 kJ mol–1 and Δ S = 300 JK–1 mol–1. The temperature at which the reaction will be in equilibrium, is Options: a 327

enthalpy - Why is it sometimes kJ only, and in other times kJ/mol? What's the difference? - Chemistry Stack Exchange

The standard enthalpy of formation of NH, is -46.0kJ mol"!. If the enthalpy of formation of H, from its atoms is -436kJ mol-' and that of N, is –712kJ mol-', the average

Calculated BDEs and BDEs in (kJ mol -1 ) of ortho-and meta-substituted... | Download Scientific Diagram
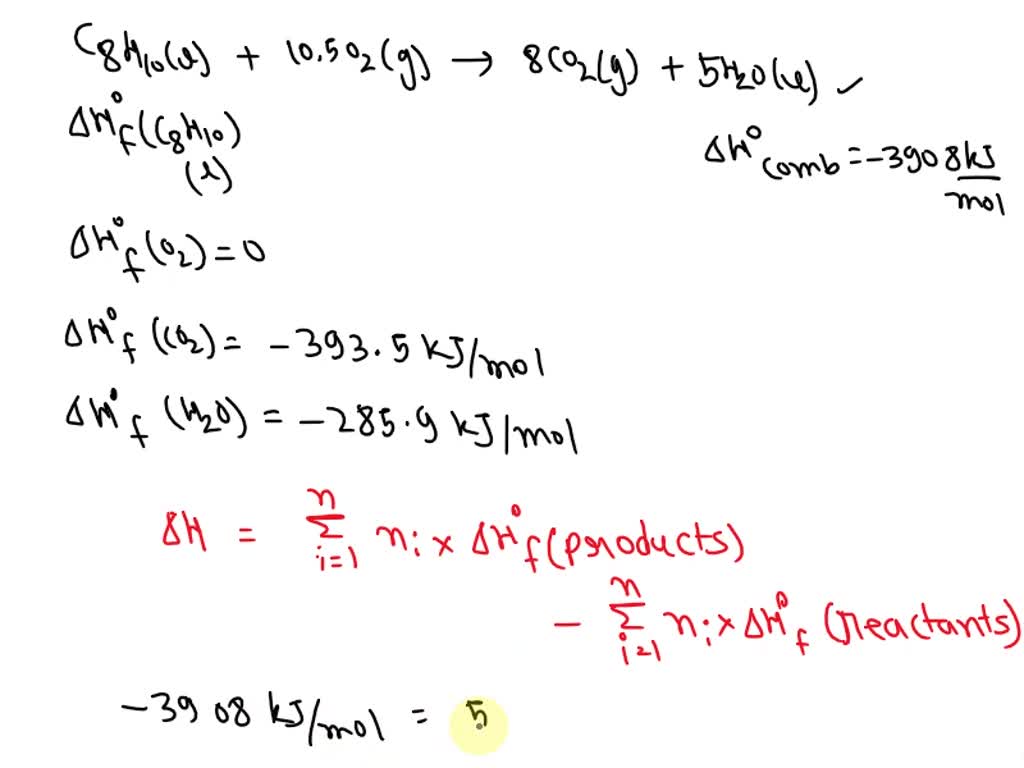
SOLVED: Physical chemistry Knowing that the standard enthalpy of combustion for xylene, CgH10(I), is -3908 kJ mol-1. Using HOf: H2O(l) = -285.9 kJ mol-1; CO2(g) = -393.5 kJ mol-1. CgH10(l) + 10.5O2(g)

enthalpy - Why is it sometimes kJ only, and in other times kJ/mol? What's the difference? - Chemistry Stack Exchange

43. Energy of the electron in Hydrogen atom is gives by (a) En2 kJ mol- (b)En kJ mol- (c) En = (d) En = 131.38 131.33 1313.3 _ kJ mol-1 313.13 kJ mol-

Enthalpy of formation of H,O, is: (1) +X, kJ mol-1 127-X, kJ mol (3) +X, kJ molt (4) -X, kJ mol Given that bond energies of H-Hand CHCl are 430 kJ moll

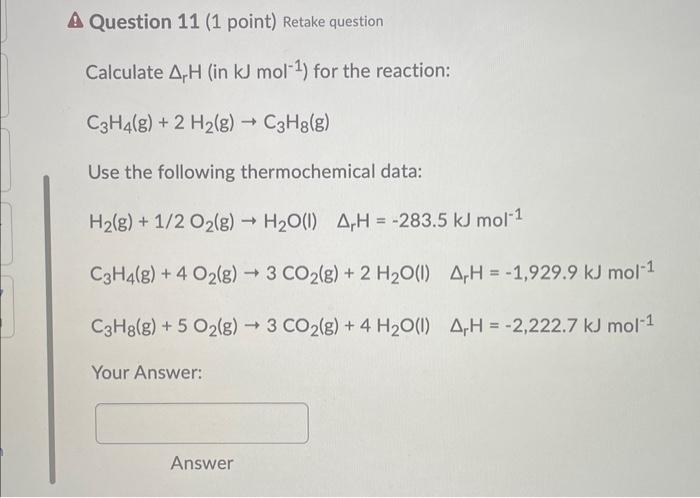




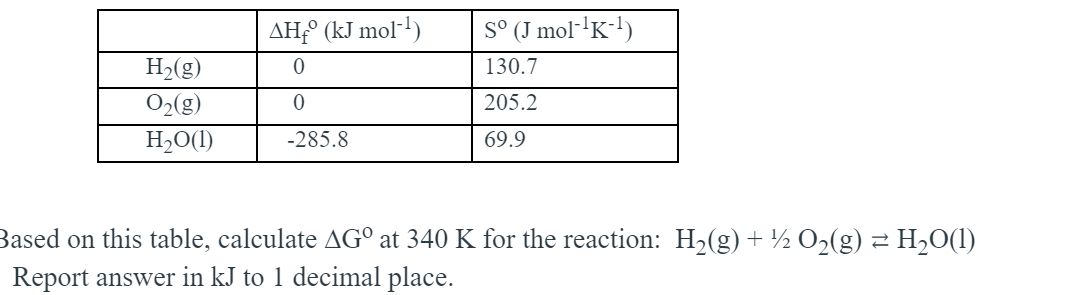

![Solved Given that Delta H degree r[H(g)] = 218.0 kJ. mol-1 | Chegg.com Solved Given that Delta H degree r[H(g)] = 218.0 kJ. mol-1 | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media/f73/f73ff219-6265-444d-89c1-620f5d2f2822/php7wff8s.png)








