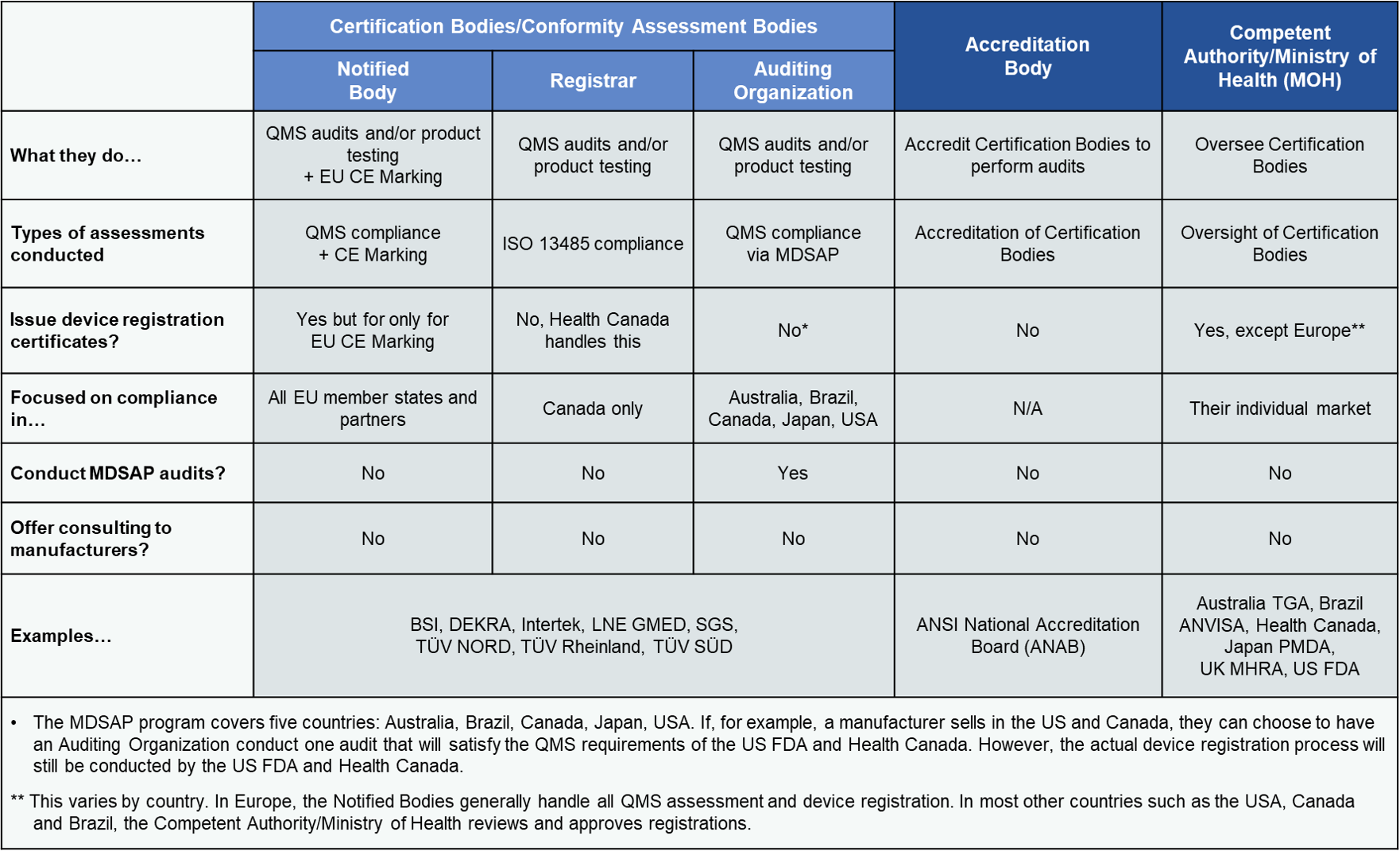
Evnia on X: "New NB designation for #IVDR! Congratulations to #Netherland's #DEKRA Product Testing & Certification for being the 5th IVDR NB. Scope: https://t.co/Vo2bgGHpX8 #medtech #biotech #invitrodiagnostics #companiondiagnostics #diagnostics ...

Which EU Notified Bodies Have Been “Designated” Under the MDR 2017/745 and IVDR 2017/746? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog